
Our Value Proposition
Medichem’s aim is to help you grow with unique APIs and FDFs while ensuring a positive customer experience. This is reflected in our value proposition:
Independently owned API and FDF
Developer and Manufacturer
MEDICHEM’S TRACK RECORD WITH INTERNATIONAL REGULATORY AGENCIES WILL HELP YOU TO GET YOUR DEVELOPMENTS APPROVED
MEDICHEM UNDERSTANDS THE IMPORTANCE OF
ON-TIME DELIVERY AND OUTSTANDING QUALITY
YOUR SUCCESS
IS OUR GOAL
WHAT MEDICHEM CAN OFFER TO HELP YOU BOOST YOUR DEVELOPMENTS AND EXPAND YOUR PRODUCT PORTFOLIO
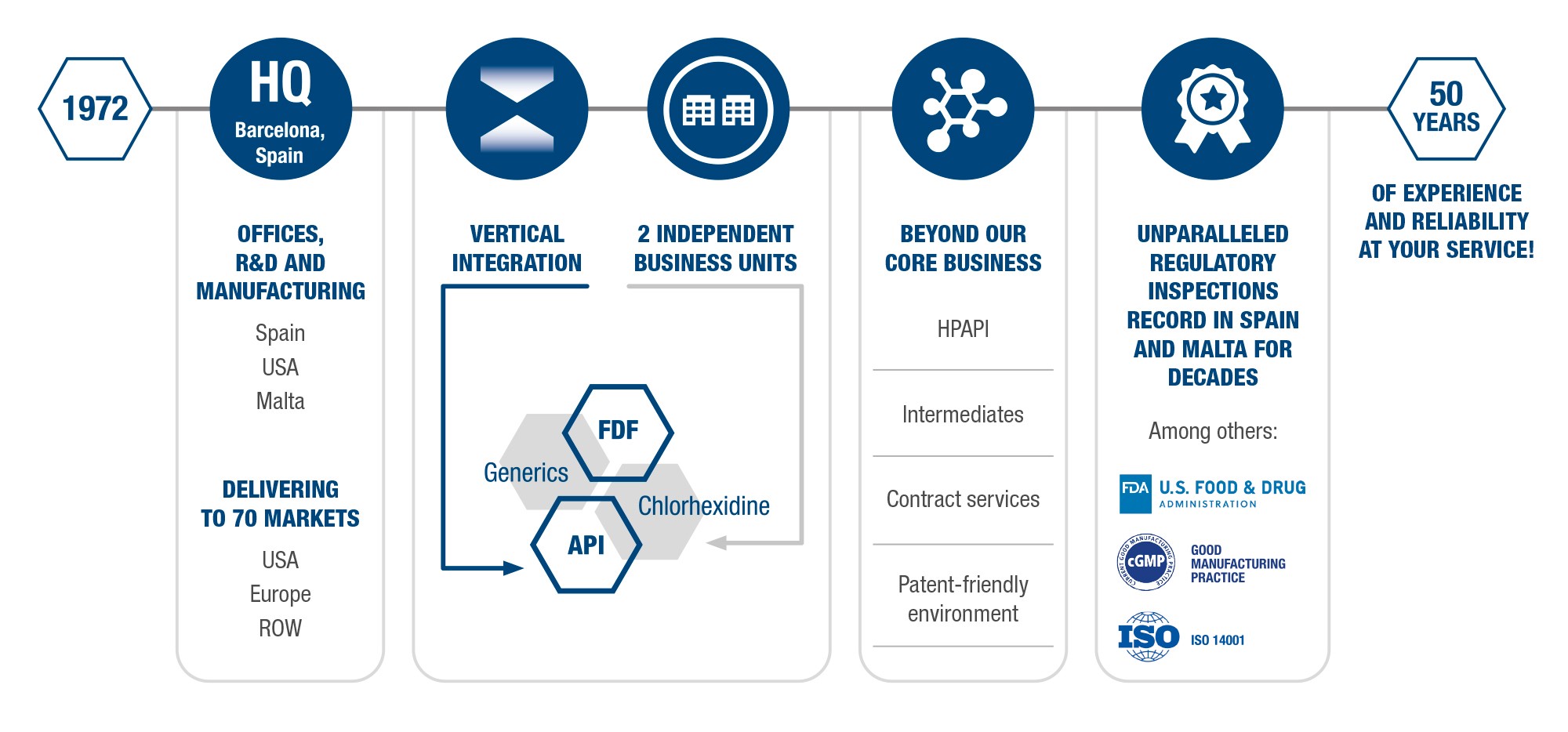
For more than half a century, Medichem has been at the forefront of innovation for its two commercial entities, Chlorhexidine and Generics, and has developed 70 value-added APIs and FDFs.
Medichem strives constantly to improve its customer service. We are present in 70 countries, with sites located in Spain, Malta and China as well as in the USA.
Medichem also goes beyond its core business by offering solutions such as custom manufacturing, HPAPIs, intermediaries and patent strategy. All this while providing an unrivalled history of regulatory.
KEY NUMBERS ABOUT MEDICHEM
Medichem is a European company founded in 1972 that offers personalized solutions (APIs & FDFs) in a vertically integrated model, having 400 international employees and 600 customers in more than 60 countries. With two manufacturing sites, 900 marketing authorizations, 16 products in development, 50 patents and patent applications and 65 available products, we are at the forefront innovation.

900
Marketing authorizations

10%
Turnover share spent on R&D

~400
Employees

50
Patents and patent applications

50
Years in operation

65
Available & unique products

2
Manufacturing sites

60
Countries distributed

16
Products in development