Medichem remains dedicated to delivering high-quality APIs and FDFs
18/04/2024
Medichem remains dedicated to delivering high-quality APIs and FDFs upholding #regulatory compliance worldwide.
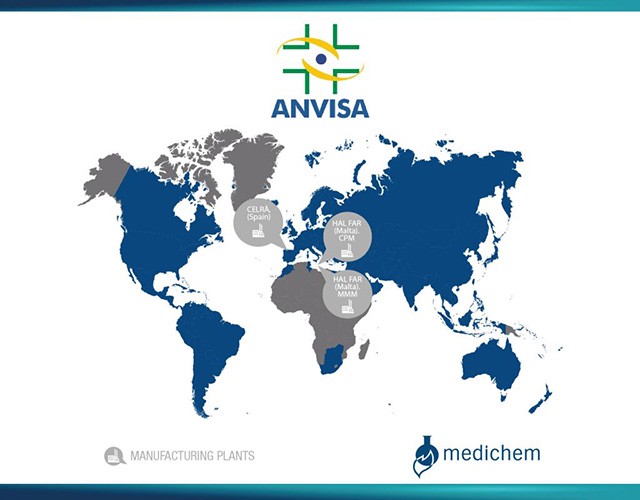
We underwent our first Brazil ANVISA (AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA) inspections at our API and FDF facilities in #Malta, in April 2023 and May 2023 respectively. Both inspections were successfully concluded, and the sites were approved.
This achievement builds upon our previous milestone in May 2022 when our Celrà site received the ANVISA GMP Certification for some of our APIs.
Excited about these achievements, we look forward to serving our #partners and customers with continued #excellence!
To find out how we can support your registration needs for these products globally, please reach out to us today.
https://lnkd.in/d3HJRGXJ


